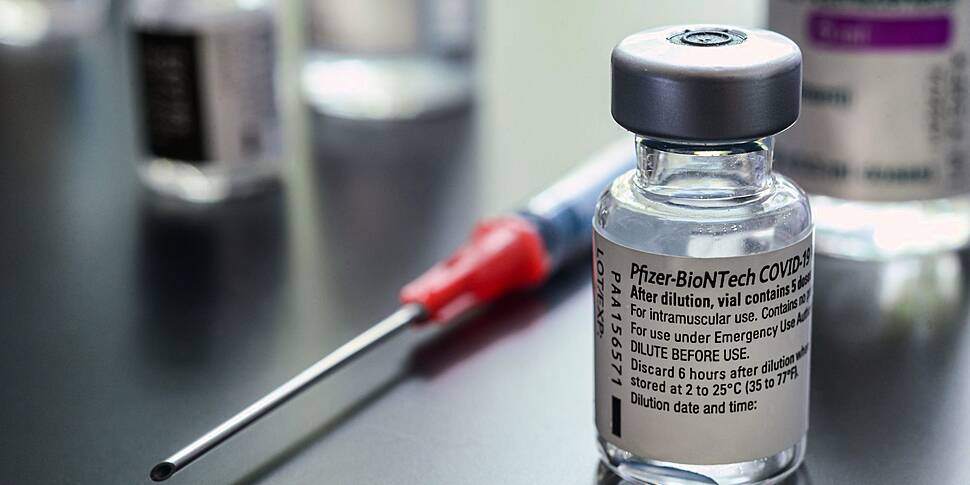
The European Medicines Agency has approved a Covid-19 vaccine for children.
‼️ EMA recommends approval of BioNTech/Pfizer’s #COVID19vaccine, Comirnaty, for children aged 5 to 11.
In this population, the dose of #Comirnaty will be lower than that used in people aged 12 and above.
Read the full press release: https://t.co/uqqBAklVvl pic.twitter.com/NZQhli4SDl
— EU Medicines Agency (@EMA_News) November 25, 2021
It's given the green light to Pfizer to allow reduced doses in 5 to 11-year-olds.
Welcome news from @EMA_News As with all our vaccination programmes we'll receive advice from NIAC and we've been working with @HSELive to be prepared to administer these vaccines in due course. https://t.co/0m1DRcJpiv
— Stephen Donnelly (@DonnellyStephen) November 25, 2021
The National Immunisation Advisory Committee will now consider the decision for Ireland.